Introducing the Electron Configuration Review Worksheet Answer Key, an indispensable guide to unraveling the mysteries of atomic structure. This comprehensive resource provides a thorough review of electron configuration concepts, empowering learners with a deeper understanding of the periodic table and its implications in chemistry and beyond.
Delving into the intricate relationship between electron configuration and chemical properties, this worksheet unravels the secrets of how atomic structure governs the behavior of elements. Through detailed explanations and practice exercises, learners will gain a profound comprehension of electron configurations, their impact on chemical bonding, and their significance in various scientific disciplines.
Electron Configuration

Electron configuration refers to the distribution of electrons in different energy levels or orbitals around the nucleus of an atom. It provides a detailed description of the arrangement of electrons in an atom, which is crucial for understanding the chemical properties and behavior of elements.
The electron configuration of an element is represented using a set of quantum numbers, including the principal quantum number (n), angular momentum quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These quantum numbers describe the energy level, shape, orientation, and spin of the electron, respectively.
Examples of Electron Configurations, Electron configuration review worksheet answer key
- Hydrogen (H): 1s 1
- Helium (He): 1s 2
- Lithium (Li): 1s 22s 1
- Carbon (C): 1s 22s 22p 2
- Oxygen (O): 1s 22s 22p 4
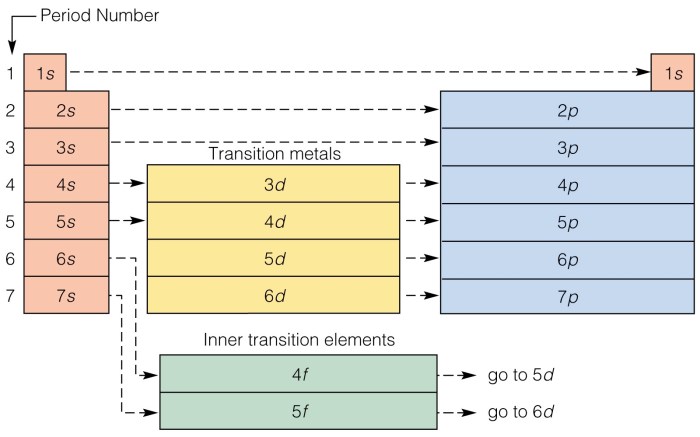
Periodic Table and Electron Configuration
The periodic table is a tabular arrangement of chemical elements organized based on their atomic number, electron configuration, and recurring chemical properties. The electron configuration of elements plays a crucial role in determining their position in the periodic table.
Elements with similar electron configurations tend to be grouped together in the periodic table. For instance, all alkali metals (Group 1) have one valence electron in the outermost energy level (ns 1), while all noble gases (Group 18) have a stable electron configuration with a full outermost energy level (ns 2np 6).
By examining the electron configuration of an element, we can predict its chemical properties. For example, elements with a full outermost energy level are typically unreactive, while those with one or a few valence electrons are highly reactive.
Worksheet Review
Electron configuration review worksheets typically cover the following key concepts:
- Quantum numbers and their significance
- Aufbau principle and Hund’s rule
- Writing electron configurations for various elements
- Predicting chemical properties based on electron configuration
Answers to the worksheet questions should include:
- Detailed explanations of the concepts being tested
- Correctly written electron configurations for the given elements
- Logical reasoning and evidence to support predictions
Practice Exercises
Practice exercises can reinforce the understanding of electron configuration by providing opportunities to:
- Write electron configurations for a range of elements
- Determine the number of valence electrons for a given element
- Predict the chemical properties of elements based on their electron configurations
Answer keys for the practice exercises should provide:
- Correct answers to all questions
- Explanations for the reasoning behind the answers
- Additional resources or references for further exploration
Applications of Electron Configuration: Electron Configuration Review Worksheet Answer Key
Electron configuration finds practical applications in various fields, including:
- Chemistry:Predicting chemical reactivity, bonding behavior, and molecular structure
- Physics:Understanding atomic spectroscopy, magnetism, and electronic properties of materials
- Materials science:Designing new materials with tailored properties, such as semiconductors and superconductors
For example, in materials science, electron configuration is used to engineer materials with specific electronic bandgaps, which are crucial for applications in solar cells, light-emitting diodes (LEDs), and transistors.
Advanced Concepts
Advanced concepts related to electron configuration include:
- Hund’s rule:Electrons in degenerate orbitals (orbitals with the same energy) tend to occupy different orbitals with parallel spins before pairing up.
- Aufbau principle:Electrons fill orbitals in the order of increasing energy, starting with the lowest energy level.
These concepts help us understand the behavior of atoms and molecules in more complex systems and are essential for advanced studies in chemistry, physics, and materials science.
FAQ
What is electron configuration?
Electron configuration refers to the distribution of electrons in different energy levels or orbitals around the nucleus of an atom.
How is electron configuration related to the periodic table?
The periodic table is organized based on the electron configurations of elements, with elements in the same group sharing similar electron configurations and chemical properties.
Why is electron configuration important?
Electron configuration plays a crucial role in determining the chemical behavior of elements, influencing their reactivity, bonding properties, and physical characteristics.